[ad_1]
Researchers have developed a stable electrolyte for transporting hydride ions at room temperature, opening doorways to safer, extra environment friendly hydrogen-based batteries and gasoline cells.
Researchers on the RIKEN Cluster for Pioneering Analysis in Japan, led by Genki Kobayashi, have achieved a big milestone in hydrogen-based power storage. They’ve efficiently created a stable electrolyte able to transporting hydride ions (H−) at room temperature. This achievement opens up new avenues for the sensible implementation of hydrogen-based solid-state batteries and gasoline cells, promising enhanced security, effectivity, and power density essential for advancing in the direction of a viable hydrogen-based power financial system. The small print of their breakthrough have been revealed within the prestigious journal Superior Power Supplies.
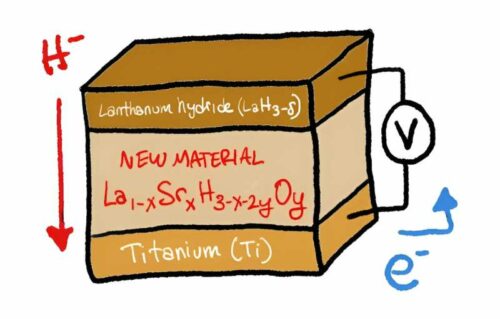
Hydrogen-based power storage and gasoline methods have lengthy been recognised as promising options, however they face important challenges, together with security issues and effectivity points. Present hydrogen-based gasoline cells depend on proton conduction by way of a polymer membrane, necessitating fixed hydration to forestall drying out. This requirement provides complexity and price to battery and gasoline cell designs, hampering the transition to a hydrogen-based power financial system. To deal with this problem, scientists have been growing a method of conducting damaging hydride ions by way of stable supplies, significantly at room temperature. The group led by Genki Kobayashi targeted their efforts on lanthanum hydrides (LaH3-δ) as a consequence of their favorable properties, together with simple hydrogen launch and seize, excessive hydride ion conduction, and stability at temperatures under 100°C.
The primary hurdle was the fluctuation within the variety of hydrogens connected to lanthanum at room temperature, stopping environment friendly conduction—a phenomenon generally known as hydrogen non-stoichiometry. To beat this impediment, the researchers substituted a number of the lanthanum with strontium (Sr) and launched a small quantity of oxygen. This resulted in a system (La1-xSrxH3-x-2yOy) that enabled environment friendly hydride ion conduction. The group ready crystalline samples of the fabric by way of ball-milling and annealing processes, demonstrating excessive charges of hydride ion conduction at room temperature. Furthermore, they examined the fabric’s efficiency in a solid-state gasoline cell, reaching full conversion of titanium to titanium hydride (TiH2) with an optimum strontium content material of at the very least 0.2. This breakthrough minimizes hydride ion wastage.
In the long run, this growth is poised to revolutionize batteries, gasoline cells, and electrolytic cells powered by hydrogen, marking a big turning level within the subject. Shifting ahead, the researchers intention to boost efficiency and create electrode supplies able to reversible hydrogen absorption and launch, an important step in the direction of enabling recharging of batteries and environment friendly hydrogen storage, important for the widespread adoption of hydrogen-based power options.
[ad_2]
