| Oct 12, 2023 |
|
|
|
(Nanowerk Information) Regardless of its basic position in biology, and intensive research over half a century, many points of how DNA’s constructing blocks are fashioned stay unclear. Now, a global group of scientists has revealed beneficial particulars about this intricate course of. The analysis, revealed in Science (“Construction of a ribonucleotide reductase R2 protein radical”), provides insights into the novel enzyme – a extremely reactive molecule that initiates the DNA synthesis – and will pave the best way for medical and therapeutic purposes for most cancers and infectious ailments.
|
|
The group, which included researchers from Stockholm College, CNRS-College of Toulouse, the Division of Vitality’s SLAC Nationwide Accelerator Laboratory and Lawrence Berkeley Nationwide Laboratory and a number of other different establishments mixed their experience to crack open the mysteries of ribonucleotide reductases (RNRs), a novel set of enzymes that produce the DNA constructing blocks.
|
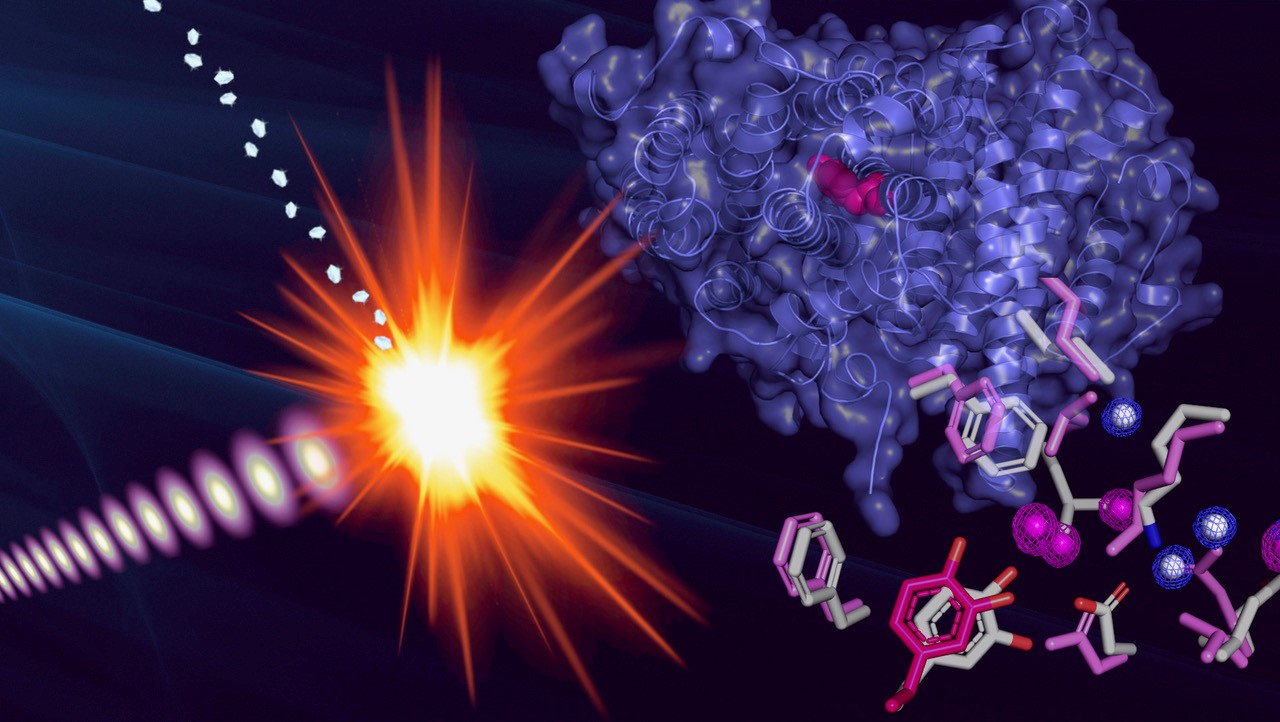 |
| The construction of the protein radical was decided by exposing microcrystals of the novel protein to extraordinarily brief and intense pulses from an X-ray laser. (Picture: Martin Högbom, Stockholm College)
|
|
RNRs have puzzled scientists for many years. They generate free radicals, that are a sort of molecule that may trigger injury to cells, however that are additionally important to a number of biochemical processes. Fixing the thriller of RNRs lies in understanding their lively radical state, a seemingly paradoxical phenomenon first found 50 years in the past wherein the protein is itself a radical, and thus has an odd variety of electrons.
|
|
“Having a background in chemistry, it amazed me after I discovered that enzymes used radicals,” stated Martin Högbom, a researcher at Stockholm College who led the analysis. “At the moment, the thought of figuring out what a protein radical seems like appeared even theoretically far-fetched. However this curiosity adopted me all through my scientific profession.”
|
|
Over time, many enzyme techniques have been acknowledged to make use of radical chemistry, however till now it has not been attainable to watch the construction of proteins on this reactive state resulting from their inherent sensitivity to being measured.
|
|
“We use X-rays to measure the construction of proteins, however radicals are extraordinarily delicate to radiation injury induced by these X-ray beams.” stated SLAC scientist and collaborator Roberto Alonso-Mori. “The X-rays can generate many electrons and different radicals which might nullify the protein radical state we wish to examine.”
|
|
Utilizing SLAC’s Linac Coherent Gentle Supply (LCLS) X-ray laser, the group employed a cutting-edge approach known as serial femtosecond crystallography, which permits researchers to watch proteins and different molecules on the temperature at which they’re present in nature, coupled to diffraction-before-destruction, which permits researchers to gather exact data from delicate samples within the immediate earlier than they’re blown aside by the laser. This allowed them to seize photos of the protein in its lively radical state for the primary time, offering direct perception into the way it behaves when it’s useful.
|
|
Past its foundational significance in biology, the invention has therapeutic potential since RNR is important for cell division.
|
|
Collaborator Jan Kern, a scientist at Lawrence Berkeley Nationwide Laboratory. stated “With this new methodology, we will perceive the pure management and utilization of those reactive states, providing potential developments in remedies, particularly for situations like most cancers.”
|
|
To comply with up, the researchers hope to broaden their research into different types of this enzyme.
|
|
“We goal to check different forms of ribonucleotide reductases, increasing our understanding of radical formation in several enzyme varieties,” stated collaborator Hugo Lebrette, a former postdoctoral researcher at Stockholm College and now a analysis group chief on the CNRS-College of Toulouse.
|
|
“Evaluating these may present insights into focusing on particular enzymes in related organisms,” added collaborator Vivek Srinivas, postdoctoral researcher at Stockholm College, “This could open the door to observing completely different proteins of their lively types, with the hope that this will reshape illness remedy strategies.”
|
|
Whereas the info assortment itself was executed inside an hour, the muse for this milestone was laid over a number of many years, marked by meticulous groundwork, identification of appropriate mannequin techniques, and pattern preparation.
|
|
“My fascination with protein radicals started almost 30 years in the past throughout my undergraduate research,” Högbom stated. “The idea of an enzyme producing and sustaining a radical was a revelation. Our objective is to know this protein household comprehensively, and every experiment, every paper, propels us nearer to that goal. This newest result’s a significant step ahead.”
|


